Στην πραγματικοτητα εαν διαβασει κανεις το γραμμα εγκρισης απο FDA στις ΗΠΑ, δεν δινει απολυτη εγκριση οπως το παρουσιαζουν. FDA χρησημοποιει την λεξη indicated (3η παραγραφο στην πρωτη σελιδα) που σημαινει ” ενδεικνυται , δεν χρησημοποιει την λεξη “confirm” oπως σε καθε εγκριση που δινει. Απλα με το γραμμα εγκρισης η FDA, δινει την αδεια να κατασκευαζουν το εμβολιο που ενδεικνυται να ειναι αποτελεσματικο.
Εδω το Pdf August 23, 2021 Approval Letter – Comirnaty
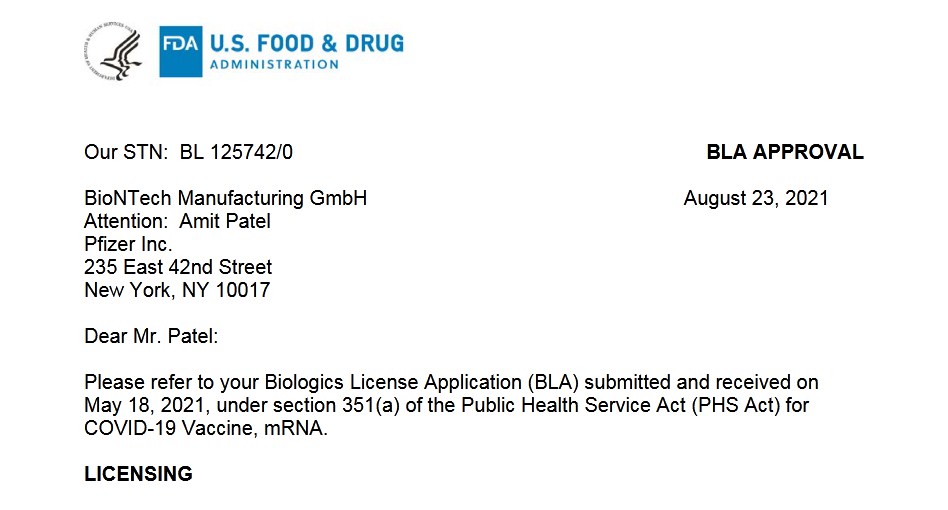
Our STN: BL 125742/0 BLA APPROVAL
BioNTech Manufacturing GmbH August 23, 2021 Attention: Amit Patel
Pfizer Inc.
235 East 42nd Street
New York, NY 10017
Dear Mr. Patel:
Please refer to your Biologics License Application (BLA) submitted and received on May 18, 2021, under section 351(a) of the Public Health Service Act (PHS Act) for COVID-19 Vaccine, mRNA.
LICENSING
We are issuing Department of Health and Human Services U.S. License No. 2229 to BioNTech Manufacturing GmbH, Mainz, Germany, under the provisions of section 351(a) of the PHS Act controlling the manufacture and sale of biological products. The license authorizes you to introduce or deliver for introduction into interstate commerce, those products for which your company has demonstrated compliance with establishment and product standards.
Under this license, you are authorized to manufacture the product, COVID-19 Vaccine, mRNA, which is indicated for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 16 years of age and older.
The review of this product was associated with the following National Clinical Trial (NCT) numbers: NCT04368728 and NCT04380701.
MANUFACTURING LOCATIONS
Under this license, you are approved to manufacture COVID-19 Vaccine, mRNA drug
substance at
The final formulated product will be manufactured, filled,
(b) (4)
(b) (4)
labeled and packaged at Pfizer
(b) (4)
. The diluent, 0.9% Sodium Chloride Injection, USP, will be manufactured at
U.S. Food & Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 w ww.fda.gov
Page 2 – STN BL 125742/0 – Elisa Harkins
You may label your product with the proprietary name, COMIRNATY, and market it in 2.0 mL glass vials, in packages of 25 and 195 vials.
We did not refer your application to the Vaccines and Related Biological Products Advisory Committee because our review of information submitted in your BLA, including the clinical study design and trial results, did not raise concerns or controversial issues that would have benefited from an advisory committee discussion.
DATING PERIOD
The dating period for COVID-19 Vaccine, mRNA shall be 9 months from the date of manufacture when stored between -90ºC to -60ºC (-130ºF to -76ºF). The date of manufacture shall be no later than the date of final sterile filtration of the formulated (b) (4)
drug product (at , the date of manufacture is defined as the date of sterile filtration for the final drug product; at (b) (4)(b) (4)
Pfizer , it is defined as the date of the
Following the final sterile filtration,
, no
(b) (4)
reprocessing/reworking is allowed without prior approval from the Agency. The dating (b) (4) (b) (4)
period for your drug substance shall be when stored at We have approved the stability protocols in your license application for the purpose of extending the expiration dating period of your drug substance and drug product under 21 CFR 601.12.
FDA LOT RELEASE
Please submit final container samples of the product in final containers together with protocols showing results of all applicable tests. You may not distribute any lots of product until you receive a notification of release from the Director, Center for Biologics Evaluation and Research (CBER).
BIOLOGICAL PRODUCT DEVIATIONS
You must submit reports of biological product deviations under 21 CFR 600.14. You should identify and investigate all manufacturing deviations promptly, including those associated with processing, testing, packaging, labeling, storage, holding and distribution. If the deviation involves a distributed product, may affect the safety, purity, or potency of the product, and meets the other criteria in the regulation, you must submit a report on Form FDA 3486 to the Director, Office of Compliance and Biologics Quality, electronically through the eBPDR web application or at the address below. Links for the instructions on completing the electronic form (eBPDR) may be found on CBER’s web site at https://www.fda.gov/vaccines-blood-biologics/report-problem-center biologics-evaluation-research/biological-product-deviations:
Food and Drug Administration
Center for Biologics Evaluation and Research
Document Control Center
Page 3 – STN BL 125742/0 – Elisa Harkins
10903 New Hampshire Ave.
WO71-G112
Silver Spring, MD 20993-0002
MANUFACTURING CHANGES
You must submit information to your BLA for our review and written approval under 21 CFR 601.12 for any changes in, including but not limited to, the manufacturing, testing, packaging or labeling of COVID-19 Vaccine, mRNA, or in the manufacturing facilities.
LABELING
We hereby approve the draft content of labeling including Package Insert, submitted under amendment 74, dated August 21, 2021, and the draft carton and container labels submitted under amendment 63, dated August 19, 2021.
CONTENT OF LABELING
As soon as possible, but no later than 14 days from the date of this letter, please submit the final content of labeling (21 CFR 601.14) in Structured Product Labeling (SPL) format via the FDA automated drug registration and listing system, (eLIST) as described at http://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabeling/ default.htm. Content of labeling must be identical to the Package Insert submitted on August 21, 2021. Information on submitting SPL files using eLIST may be found in the guidance for industry SPL Standard for Content of Labeling Technical Qs and As at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guida nces/UCM072392.pdf.
The SPL will be accessible via publicly available labeling repositories. CARTON AND CONTAINER LABELS
Please electronically submit final printed carton and container labels identical to the carton and container labels submitted on August 19, 2021, according to the guidance for industry Providing Regulatory Submissions in Electronic Format — Certain Human Pharmaceutical Product Applications and Related Submissions Using the eCTD Specifications at https://www.fda.gov/regulatory-information/search-fda-guidance documents/providing-regulatory-submissions-electronic-format-certain-human pharmaceutical-product-applications.
All final labeling should be submitted as Product Correspondence to this BLA STN BL 125742 at the time of use and include implementation information on Form FDA 356h.
ADVERTISING AND PROMOTIONAL LABELING
Page 4 – STN BL 125742/0 – Elisa Harkins
You may submit two draft copies of the proposed introductory advertising and promotional labeling with Form FDA 2253 to the Advertising and Promotional Labeling Branch at the following address:
Food and Drug Administration
Center for Biologics Evaluation and Research
Document Control Center
10903 New Hampshire Ave.
WO71-G112
Silver Spring, MD 20993-0002
You must submit copies of your final advertising and promotional labeling at the time of initial dissemination or publication, accompanied by Form FDA 2253 (21 CFR 601.12(f)(4)).
All promotional claims must be consistent with and not contrary to approved labeling. You should not make a comparative promotional claim or claim of superiority over other products unless you have substantial evidence or substantial clinical experience to support such claims (21 CFR 202.1(e)(6)).
ADVERSE EVENT REPORTING
You must submit adverse experience reports in accordance with the adverse experience reporting requirements for licensed biological products (21 CFR 600.80), and you must submit distribution reports at monthly intervals as described in 21 CFR 600.81. For information on adverse experience reporting, please refer to the guidance for industry Providing Submissions in Electronic Format —Postmarketing Safety Reports for Vaccines at https://www.fda.gov/regulatory-information/search-fda guidance-documents/providing-submissions-electronic-format-postmarketing-safety reports-vaccines. For information on distribution reporting, please refer to the guidance for industry Electronic Submission of Lot Distribution Reports at
http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation /Post-MarketActivities/LotReleases/ucm061966.htm.
PEDIATRIC REQUIREMENTS
Under the Pediatric Research Equity Act (PREA) (21 U.S.C. 355c), all applications for new active ingredients, new indications, new dosage forms, new dosing regimens, or new routes of administration are required to contain an assessment of the safety and effectiveness of the product for the claimed indication in pediatric patients unless this requirement is waived, deferred, or inapplicable.
We are deferring submission of your pediatric studies for ages younger than 16 years for this application because this product is ready for approval for use in individuals 16 years of age and older, and the pediatric studies for younger ages have not been completed.
Page 5 – STN BL 125742/0 – Elisa Harkins
Your deferred pediatric studies required under section 505B(a) of the Federal Food, Drug, and Cosmetic Act (FDCA) are required postmarketing studies. The status of these postmarketing studies must be reported according to 21 CFR 601.28 and section 505B(a)(4)(C) of the FDCA. In addition, section 506B of the FDCA and 21 CFR 601.70 require you to report annually on the status of any postmarketing commitments or required studies or clinical trials.
Label your annual report as an “Annual Status Report of Postmarketing Study Requirement/Commitments” and submit it to the FDA each year within 60 calendar days of the anniversary date of this letter until all Requirements and Commitments subject to the reporting requirements under section 506B of the FDCA are released or fulfilled. These required studies are listed below:
1. Deferred pediatric Study C4591001 to evaluate the safety and effectiveness of COMIRNATY in children 12 years through 15 years of age.
Final Protocol Submission: October 7, 2020
Study Completion: May 31, 2023
Final Report Submission: October 31, 2023
2. Deferred pediatric Study C4591007 to evaluate the safety and effectiveness of COMIRNATY in infants and children 6 months to <12 years of age.
Final Protocol Submission: February 8, 2021
Study Completion: November 30, 2023
Final Report Submission: May 31, 2024
3. Deferred pediatric Study C4591023 to evaluate the safety and effectiveness of COMIRNATY in infants <6 months of age.
Final Protocol Submission: January 31, 2022
Study Completion: July 31, 2024
Final Report Submission: October 31, 2024
Submit the protocols to your IND 19736, with a cross-reference letter to this BLA STN BL 125742 explaining that these protocols were submitted to the IND. Please refer to the PMR sequential number for each study/clinical trial and the submission number as shown in this letter.
Submit final study reports to this BLA STN BL 125742. In order for your PREA PMRs to be considered fulfilled, you must submit and receive approval of an efficacy or a labeling
Page 6 – STN BL 125742/0 – Elisa Harkins
supplement. For administrative purposes, all submissions related to these required pediatric postmarketing studies must be clearly designated as:
• Required Pediatric Assessment(s)
We note that you have fulfilled the pediatric study requirement for ages 16 through 17 years for this application.
POSTMARKETING REQUIREMENTS UNDER SECTION 505(o)
Section 505(o) of the Federal Food, Drug, and Cosmetic Act (FDCA) authorizes FDA to require holders of approved drug and biological product applications to conduct postmarketing studies and clinical trials for certain purposes, if FDA makes certain findings required by the statute (section 505(o)(3)(A), 21 U.S.C. 355(o)(3)(A)).
We have determined that an analysis of spontaneous postmarketing adverse events reported under section 505(k)(1) of the FDCA will not be sufficient to assess known serious risks of myocarditis and pericarditis and identify an unexpected serious risk of subclinical myocarditis.
Furthermore, the pharmacovigilance system that FDA is required to maintain under section 505(k)(3) of the FDCA is not sufficient to assess these serious risks.
Therefore, based on appropriate scientific data, we have determined that you are required to conduct the following studies:
4. Study C4591009, entitled “A Non-Interventional Post-Approval Safety Study of the Pfizer-BioNTech COVID-19 mRNA Vaccine in the United States,” to evaluate the occurrence of myocarditis and pericarditis following administration of COMIRNATY.
We acknowledge the timetable you submitted on August 21, 2021, which states that you will conduct this study according to the following schedule:
Final Protocol Submission: August 31, 2021
Monitoring Report Submission: October 31, 2022
Interim Report Submission: October 31, 2023
Study Completion: June 30, 2025
Final Report Submission: October 31, 2025
5. Study C4591021, entitled “Post Conditional Approval Active Surveillance Study Among Individuals in Europe Receiving the Pfizer-BioNTech Coronavirus
Page 7 – STN BL 125742/0 – Elisa Harkins
Disease 2019 (COVID-19) Vaccine,” to evaluate the occurrence of myocarditis and pericarditis following administration of COMIRNATY.
We acknowledge the timetable you submitted on August 21, 2021, which states that you will conduct this study according to the following schedule:
Final Protocol Submission: August 11, 2021
Progress Report Submission: September 30, 2021
Interim Report 1 Submission: March 31, 2022
Interim Report 2 Submission: September 30, 2022
Interim Report 3 Submission: March 31, 2023
Interim Report 4 Submission: September 30, 2023
Interim Report 5 Submission: March 31, 2024
Study Completion: March 31, 2024
Final Report Submission: September 30, 2024
6. Study C4591021 substudy to describe the natural history of myocarditis and pericarditis following administration of COMIRNATY.
We acknowledge the timetable you submitted on August 21, 2021, which states that you will conduct this study according to the following schedule:
Final Protocol Submission: January 31, 2022
Study Completion: March 31, 2024
Final Report Submission: September 30, 2024
7. Study C4591036, a prospective cohort study with at least 5 years of follow-up for potential long-term sequelae of myocarditis after vaccination (in collaboration with Pediatric Heart Network).
We acknowledge the timetable you submitted on August 21, 2021, which states that you will conduct this study according to the following schedule:
Final Protocol Submission: November 30, 2021
Study Completion: December 31, 2026
Page 8 – STN BL 125742/0 – Elisa Harkins
Final Report Submission: May 31, 2027
8. Study C4591007 substudy to prospectively assess the incidence of subclinical myocarditis following administration of the second dose of COMIRNATY in a subset of participants 5 through 15 years of age.
We acknowledge the timetable you submitted on August 21, 2021, which states that you will conduct this assessment according to the following schedule:
Final Protocol Submission: September 30, 2021
Study Completion: November 30, 2023
Final Report Submission: May 31, 2024
9. Study C4591031 substudy to prospectively assess the incidence of subclinical myocarditis following administration of a third dose of COMIRNATY in a subset of participants 16 to 30 years of age.
We acknowledge the timetable you submitted on August 21, 2021, which states that you will conduct this study according to the following schedule:
Final Protocol Submission: November 30, 2021
Study Completion: June 30, 2022
Final Report Submission: December 31, 2022
Please submit the protocols to your IND 19736, with a cross-reference letter to this BLA STN BL 125742 explaining that these protocols were submitted to the IND. Please refer to the PMR sequential number for each study/clinical trial and the submission number as shown in this letter.
Please submit final study reports to the BLA. If the information in the final study report supports a change in the label, the final study report must be submitted as a supplement to this BLA STN BL 125742. For administrative purposes, all submissions related to these postmarketing studies required under section 505(o) must be submitted to this BLA and be clearly designated as:
• Required Postmarketing Correspondence under Section 505(o) • Required Postmarketing Final Report under Section 505(o)
• Supplement contains Required Postmarketing Final Report under Section 505(o)
Section 505(o)(3)(E)(ii) of the FDCA requires you to report periodically on the status of any study or clinical trial required under this section. This section also requires you to periodically report to FDA on the status of any study or clinical trial otherwise
Page 9 – STN BL 125742/0 – Elisa Harkins
undertaken to investigate a safety issue. In addition, section 506B of the FDCA and 21 CFR 601.70 require you to report annually on the status of any postmarketing commitments or required studies or clinical trials.
You must describe the status in an annual report on postmarketing studies for this product. Label your annual report as an Annual Status Report of Postmarketing Requirements/Commitments and submit it to the FDA each year within 60 calendar days of the anniversary date of this letter until all Requirements and Commitments subject to the reporting requirements of section 506B of the FDCA are fulfilled or released. The status report for each study should include:
• the sequential number for each study as shown in this letter;
• information to identify and describe the postmarketing requirement; • the original milestone schedule for the requirement;
• the revised milestone schedule for the requirement, if appropriate; • the current status of the requirement (i.e., pending, ongoing, delayed, terminated, or submitted); and,
• an explanation of the status for the study or clinical trial. The explanation should include how the study is progressing in reference to the original projected schedule, including, the patient accrual rate (i.e., number enrolled to date and the total planned enrollment).
As described in 21 CFR 601.70(e), we may publicly disclose information regarding these postmarketing studies on our website at http://www.fda.gov/Drugs/Guidance ComplianceRegulatoryInformation/Post-marketingPhaseIVCommitments/default.htm.
We will consider the submission of your annual report under section 506B of the FDCA and 21 CFR 601.70 to satisfy the periodic reporting requirement under section 505(o)(3)(E)(ii) provided that you include the elements listed in section 505(o) and 21 CFR 601.70. We remind you that to comply with section 505(o), your annual report must also include a report on the status of any study or clinical trial otherwise undertaken to investigate a safety issue. Failure to periodically report on the status of studies or clinical trials required under section 505(o) may be a violation of FDCA section 505(o)(3)(E)(ii) and could result in regulatory action.
POSTMARKETING COMMITMENTS SUBJECT TO REPORTING REQUIREMENTS UNDER SECTION 506B
We acknowledge your written commitments as described in your letter of August 21, 2021 as outlined below:
10. Study C4591022, entitled “Pfizer-BioNTech COVID-19 Vaccine Exposure during Pregnancy: A Non-Interventional Post-Approval Safety Study of Pregnancy and Infant Outcomes in the Organization of Teratology Information Specialists (OTIS)/MotherToBaby Pregnancy Registry.”
Final Protocol Submission: July 1, 2021
Page 10 – STN BL 125742/0 – Elisa Harkins
Study Completion: June 30, 2025
Final Report Submission: December 31, 2025
11. Study C4591007 substudy to evaluate the immunogenicity and safety of lower dose levels of COMIRNATY in individuals 12 through <30 years of age.
Final Protocol Submission: September 30, 2021
Study Completion: November 30, 2023
Final Report Submission: May 31, 2024
12. Study C4591012, entitled “Post-emergency Use Authorization Active Safety Surveillance Study Among Individuals in the Veteran’s Affairs Health System Receiving Pfizer-BioNTech Coronavirus Disease 2019 (COVID-19) Vaccine.”
Final Protocol Submission: January 29, 2021
Study Completion: June 30, 2023
Final Report Submission: December 31, 2023
13.Study C4591014, entitled “Pfizer-BioNTech COVID-19 BNT162b2 Vaccine Effectiveness Study - Kaiser Permanente Southern California.”
Final Protocol Submission: March 22, 2021
Study Completion: December 31, 2022
Final Report Submission: June 30, 2023
Please submit clinical protocols to your IND 19736, and a cross-reference letter to this BLA STN BL 125742 explaining that these protocols were submitted to the IND. Please refer to the PMC sequential number for each study/clinical trial and the submission number as shown in this letter.
If the information in the final study report supports a change in the label, the final study report must be submitted as a supplement. Please use the following designators to prominently label all submissions, including supplements, relating to these postmarketing study commitments as appropriate:
• Postmarketing Commitment – Correspondence Study Update • Postmarketing Commitment – Final Study Report
• Supplement contains Postmarketing Commitment – Final Study Report
Page 11 – STN BL 125742/0 – Elisa Harkins
For each postmarketing study subject to the reporting requirements of 21 CFR 601.70, you must describe the status in an annual report on postmarketing studies for this product. Label your annual report as an Annual Status Report of Postmarketing Requirements/Commitments and submit it to the FDA each year within 60 calendar days of the anniversary date of this letter until all Requirements and Commitments subject to the reporting requirements of section 506B of the FDCA are fulfilled or released. The status report for each study should include:
• the sequential number for each study as shown in this letter;
• information to identify and describe the postmarketing commitment; • the original schedule for the commitment;
• the status of the commitment (i.e., pending, ongoing, delayed, terminated, or submitted); and,
• an explanation of the status including, for clinical studies, the patient accrual rate (i.e., number enrolled to date and the total planned enrollment).
As described in 21 CFR 601.70(e), we may publicly disclose information regarding these postmarketing studies on our website at http://www.fda.gov/Drugs/Guidance ComplianceRegulatoryInformation/Post-marketingPhaseIVCommitments/default.htm.
POST APPROVAL FEEDBACK MEETING
New biological products qualify for a post approval feedback meeting. Such meetings are used to discuss the quality of the application and to evaluate the communication process during drug development and marketing application review. The purpose is to learn from successful aspects of the review process and to identify areas that could benefit from improvement. If you would like to have such a meeting with us, please contact the Regulatory Project Manager for this application.
Sincerely,
Mary A. Malarkey Marion F. Gruber, PhD Director Director
Office of Compliance Office of Vaccines and Biologics Quality Research and Review Center for Biologics Center for Biologics Evaluation and Research Evaluation and Research

Under this license, you are approved to manufacture COVID-19 Vaccine, mRNA drug
Εντάξει ρε παιδιά. Όλοι μας έχουμε ξενερώσει με την τροπή των γεγονότων αλλά αγγλικά ξέρουμε:
approved to manufatcure σημαίνει: σας εγκρίνετε να παρασκευάσετε… το ενδείκνυται αναφέρεται στην ικανότητα του εμβολίου περι ανοσοποίησης. Για την κυκλοφορία του όμως είναι ξεκάθαρο το “σας εγκρίνετε”… ελπίζω κάτι να μεσολαβήσει και να σταματήσει όλη αυτή η παράνοια που προφανώς επιφέρει αμύθητα ποσά σε τόσους
Είναι απάτη. Υπάρχει και δεύτερο έγγραφο με ίδια ημ/νια: https://www.fda.gov/media/150386/download
Συγκεκριμένα, αυτό που παρουσιάζεται εδώ αφορά προϊόν με την ονομασία COMIRNATY, το οποίο ΔΕΝ υπάρχει & ΔΕΝ βρίσκεται καν σε παραγωγή. Στο δεύτερο έγγραφο επεκτείνουν την άδεια έκτακτης χρήσης και για παιδιά 16 και άνω για το πειραματικό εμβόλιο που όλοι γνωρίζουμε. Με λίγα λόγια, η αδειοδότηση του πρώτου είναι fake. Έχει γίνει σάλος, ειδικά στις ΗΠΑ.
Πέρα από όλα αυτά, το ότι αδειοδοτείται ένα εμβόλιο ΔΕΝ σημαίνει ότι μπορεί να επιβληθεί υποχρεωτικά η χορήγηση του, ούτε νομιμοποιεί τους εκβιασμούς, τις απειλές και τις διώξεις, ούτε δικαιολογεί την παραβίαση του Συντάγματος, των Διεθνών Συνθηκών και θεμελιωδών δικαιωμάτων των πολιτών. Και εννοείται ότι η άδεια δεν το κάνει ασφαλές, γνωρίζουμε αρκετά από αυτά που προκαλεί για να μην το εμπιστευόμαστε.
Γυρίστε τους το σανό πίσω, είναι ληγμένος !!!